News
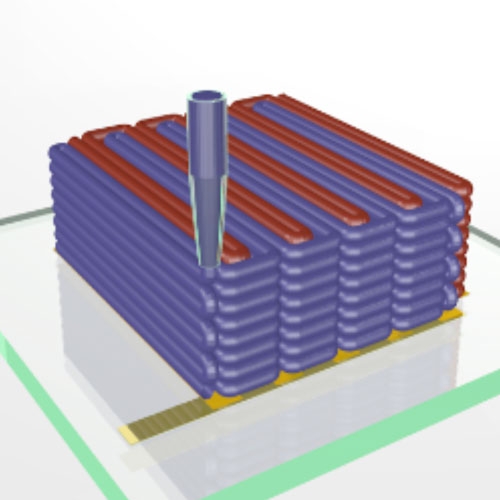
3D printing can now be used to print lithium-ion microbatteries the size of a grain of sand. (Illustration courtesy of Jennifer A. Lewis.)
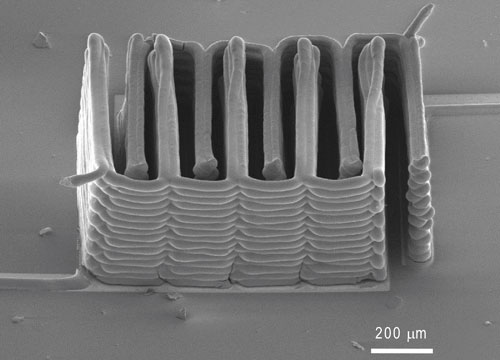
A research team from Harvard University and the University of Illinois at Urbana-Champaign has demonstrated the ability to 3D print a battery. This image shows the interlaced stack of electrodes that were printed layer by layer to create the working anode and cathode of a microbattery. (SEM image courtesy of Jennifer A. Lewis.)
Cambridge, Mass. – June 18, 2013 – 3D printing can now be used to print lithium-ion microbatteries the size of a grain of sand. The printed microbatteries could supply electricity to tiny devices in fields from medicine to communications, including many that have lingered on lab benches for lack of a battery small enough to fit the device, yet provide enough stored energy to power them.
To make the microbatteries, a team based at Harvard University and the University of Illinois at Urbana-Champaign printed precisely interlaced stacks of tiny battery electrodes, each less than the width of a human hair.
“Not only did we demonstrate for the first time that we can 3D-print a battery; we demonstrated it in the most rigorous way,” said Jennifer A. Lewis, senior author of the study, who is also the Hansjörg Wyss Professor of Biologically Inspired Engineering at the Harvard School of Engineering and Applied Sciences (SEAS), and a Core Faculty Member of the Wyss Institute for Biologically Inspired Engineering at Harvard University. Lewis led the project in her prior position at the University of Illinois at Urbana-Champaign, in collaboration with co-author Shen Dillon, an Assistant Professor of Materials Science and Engineering there.
The results have been published online in the journal Advanced Materials.
In recent years engineers have invented many miniaturized devices, including medical implants, flying insect-like robots, and tiny cameras and microphones that fit on a pair of glasses. But often the batteries that power them are as large or larger than the devices themselves, which defeats the purpose of building small.
To get around this problem, manufacturers have traditionally deposited thin films of solid materials to build the electrodes. However, due to their ultra-thin design, these solid-state micro-batteries do not pack sufficient energy to power tomorrow’s miniaturized devices.
The scientists realized they could pack more energy if they could create stacks of tightly interlaced, ultrathin electrodes that were built out of plane. For this they turned to 3D printing. 3D printers follow instructions from three-dimensional computer drawings, depositing successive layers of material—inks—to build a physical object from the ground up, much like stacking a deck of cards one at a time. The technique is used in a range of fields, from producing crowns in dental labs to rapid prototyping of aerospace, automotive, and consumer goods. Lewis’ group has greatly expanded the capabilities of 3D printing. They have designed a broad range of functional inks—inks with useful chemical and electrical properties. And they have used those inks with their custom-built 3D printers to create precise structures with the electronic, optical, mechanical, or biologically relevant properties they want.
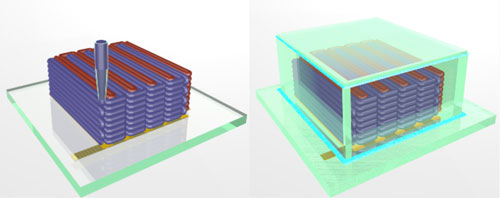
To create the microbattery, a custom-built 3D printer extrudes special inks through a nozzle narrower than a human hair. Those inks solidify to create the battery’s anode (red) and cathode (purple), layer by layer. A case (green) then encloses the electrodes and the electrolyte solution is added to create a working microbattery. (Illustration courtesy of Jennifer A. Lewis.)
To print 3D electrodes, Lewis’ group first created and tested several specialized inks. Unlike the ink in an office inkjet printer, which comes out as droplets of liquid that wet the page, the inks developed for extrusion-based 3D printing must fulfill two difficult requirements. They must exit fine nozzles like toothpaste from a tube, and they must immediately harden into their final form.
In this case, the inks also had to function as electrochemically active materials to create working anodes and cathodes, and they had to harden into layers that are as narrow as those produced by thin-film manufacturing methods. To accomplish these goals, the researchers created an ink for the anode with nanoparticles of one lithium metal oxide compound, and an ink for the cathode from nanoparticles of another. The printer deposited the inks onto the teeth of two gold combs, creating a tightly interlaced stack of anodes and cathodes. Then the researchers packaged the electrodes into a tiny container and filled it with an electrolyte solution to complete the battery.
Next, they measured how much energy could be packed into the tiny batteries, how much power they could deliver, and how long they held a charge. “The electrochemical performance is comparable to commercial batteries in terms of charge and discharge rate, cycle life and energy densities. We’re just able to achieve this on a much smaller scale,” Dillon said.
“Jennifer’s innovative microbattery ink designs dramatically expand the practical uses of 3D printing, and simultaneously open up entirely new possibilities for miniaturization of all types of devices, both medical and non-medical. It’s tremendously exciting,” said Wyss Founding Director Donald Ingber, who is also a Professor of Bioengineering at Harvard SEAS.
The work was supported by the National Science Foundation and the DOE Energy Frontier Research Center on Light-Material Interactions in Energy Conversion. Lewis and Dillon collaborated with lead author Ke Sun, a graduate student in Materials Science and Engineering at the University of Illinois at Urbana-Champaign; Teng-Sing Wei, a graduate student at Harvard SEAS; Bok Yeop Ahn, a Senior Research Scientist at the Wyss Institute and SEAS; and Jung Yoon Seo, a visiting scientist in the Lewis group, from the Korea Advanced Institute of Science and Technology.
PRESS CONTACTS:
Harvard School of Engineering and Applied Sciences
Caroline Perry, (617) 496-1351
Wyss Institute for Biologically Inspired Engineering at Harvard
Dan Ferber, (617) 432-1547
3D printing tiny batteries | Harvard School of Engineering and Applied Sciences
In this video, 3D printing is used to deposit a specially formulated "ink" through a fine nozzle to build a microbattery's anode layer by layer. Unlike an office inkjet printer that dispenses ink droplets onto paper, these inks are formulated to exit the nozzle like toothpaste from a tube and immediately harden into thin layers. The printed anode contains nanoparticles of a lithium metal oxide compound that provide the proper electrochemical properties.
Topics: Materials, Environment, Applied Physics
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.