News
Accordion-like features on the velvet worm's papilla are key to its wide-spraying slime attack, a new study has found. (Image courtesy of Cristiano Sampaio-Costa.)

The velvet worm has an unusual defense and predation mechanism. Shown here is the gigantic Solorzanoi; the masking tape in the background measures two inches. (Image courtesy of Cristiano Sampaio-Costa and Andrés Concha.)
Cambridge, Mass. – March 17, 2015 – The velvet worm is a slow-moving, unassuming creature. With its soft body, probing antennae, and stubby legs, it looks like a slug on stilts as it creeps along damp logs in tropical climates.
But it has a secret weapon. In the dark of night, when an unsuspecting cricket or termite crosses its path, the worm unleashes an instantaneous torrent of slime. Two fine jets of the gluey substance spray out of openings on its head, oscillating in all directions to cast a sticky net that entraps prey and stops it in its tracks.
Captivated, so to speak, by the worm’s split-second attack, researchers from Harvard School of Engineering and Applied Sciences (SEAS) and from universities in Chile, Costa Rica, and Brazil have been studying the creature from all angles. How, they asked, does such a slow, neurologically simple worm execute such a rapid and perfectly aimed movement?
By applying new insights from anatomy, mathematics, experimental physics, and fluid dynamics, they now have an answer—published today in Nature Communications—and the findings could inspire new microfluidic devices.
Imagine a large syringe equipped, at its narrow tip, with an elastic tube shaped like the neck of a bendy drinking straw. That is apparently the velvet worm’s slime-shooting apparatus, from its tail end—where the slime is produced and stored in a reservoir—to a pair of tiny nozzles called papillae on its head. Given this structure, a slow and gentle squeeze on the reservoir is all it takes to eject the slime with great speed and force. Most importantly, the shape and elasticity of the papillae ensure that as the slime exits, it sprays in all directions, like water gushing through a flailing garden hose.
“The geometry of the system allows the worm to squirt fast and cover a wide area. That’s the magic,” says lead author Andrés Concha, formerly a postdoctoral fellow at Harvard SEAS and now an assistant professor at Adolfo Ibañez University in Chile.
But it’s actually not the whole story, as Concha explains. A garden hose is much larger than the tube inside a velvet worm’s papillae. To get the flailing-hosepipe effect within such minuscule passages, which range in diameter from 50 to 200 microns, the worm relies on the elasticity and corrugated shape of its papillae. These features lower the fluid velocity necessary to shake the tube.
By identifying the features of the anatomy and material structure that enable the velvet worm to produce wide-spraying jets, the researchers have characterized a new type of flexible microfluidic system that they say could be used to produce fine droplets of liquid or fibrous nets, or to mix together several substances in laboratory or industrial settings.
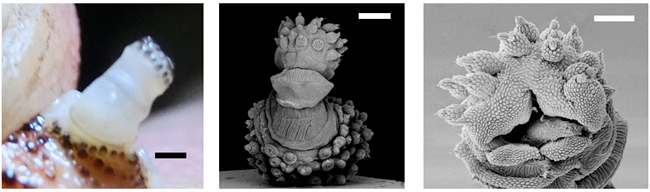
Optical and SEM images show the structure of the velvet worm's papillae. (Images courtesy of Bernal Morera-Brenes, Cristiano Sampaio-Costa and Andres Concha.)
Concha and coauthor Paula Mellado (also an assistant professor at Adolfo Ibañez University in Chile) were both Kavli Scholars at Harvard SEAS, studying topics relating to fluid dynamics, when the velvet worm project launched.
“After watching the David Attenborough film Life in the Undergrowth with some high-speed footage of the worm’s slime jet, I suggested that an elastic-hydrodynamic instability of the nozzle could be a simpler solution to creating a chaotic jet, rather than muscle control,” explains coauthor L. Mahadevan, the Lola England de Valpine Professor of Applied Mathematics at Harvard SEAS and a Professor of Organismic and Evolutionary biology and of Physics in Harvard’s Faculty of Arts and Sciences. “Our work shows that this is indeed the case, and chalks up one more example of how evolution has co-opted a simple physical principle for a behavioral response.”
Mahadevan is also a Core Faculty Member at the Wyss Institute for Biologically Inspired Engineering, a Faculty Associate of Harvard University Center for the Environment, and a member of the Kavli Institute for Bionano Science and Technology.
The unusual velvet worms present a host of new questions for future research.
“There are many cool properties of the glue that we need to explore,” Concha says. “If you put your fingers close to the mouth of the worm and you get some glue on your fingers, you wait seven seconds and you’re stuck. So one ambition is to be able to generate a synthetic glue like that, with biotechnological applications. I think there is some chemistry that we have to learn from the worm.”
The diversity of the velvet worms, which make up the genus Onychophora, also poses the question of how the squirting mechanism can have evolved to work in worms that vary greatly in size.
“That’s a great biological question,” Concha says. “By experience, we know that it works for all of these worms. Now, how they adapt the materials and the inner diameter of the hole inside the papillae, I don’t know. It’s very impressive. Even for babies, it works. You have a gigantic worm that’s eight or nine inches long and the baby is one inch, and already the mechanism is working.”
While squirting mechanisms are common among animals, anything other than a straightforward arc of liquid typically requires an active movement and some degree of control. The range of approaches to that problem within the animal kingdom requires continued research.
“Archer fish throw a jet of water, and it just follows a parabolic trajectory. Spitting cobras actively move their head to spray the poor fellow who is in front. And there are other cases—for example, spitting spiders—where the mechanism is unclear,” Concha says.
Fortunately, he has access to the venomous spitting spiders at home in Chile, where he plans to study them further. “Some biologists have posed the question, is this elasticity or is there any active mechanism? From what is in the literature up to now, I don’t have an answer, so spitting spiders are a nice thing to look forward to.”
This research was supported in part by the Comisión Nacional de Investigación Científica y Tecnológica (Conicyt) of Chile (PAI 79112004), the Fondo Nacional de Desarrollo Científico y Tecnológico (Fondecyt) of Chile (11130075, 11121397), the Universidad Nacional de Costa Rica, and the MacArthur Foundation.
Additional coauthors included Bernal Morera-Brenes, a biologist at the Universidad Nacional de Costa Rica; Cristiano Sampaio Costa, of the Universidade de Sao Paulo in Brazil; and Julian Monge-Najera, a tropical biologist at the Universidad de Costa Rica.
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
