News
In a pristine lab on Oxford street, there is a bin with compostable take-away containers, an old boot, bits of carpet and torn jackets. No, it’s not a trash can. It’s a sample container for various objects on Harvard’s campus that tested positive for the family of dangerous chemicals known as PFAS.
The samples are part of a project developed by Nicole Nishizawa, S.B. ‘19 to identify and measure PFAS-containing products on campus. The initiative was supported by Harvard’s Office for Sustainability.
What are PFAS?
Samples of materials found around campus tested for PFAS (Photo courtesy of Eliza Grinnell/Harvard SEAS)
Ever stop to wonder how a water-repellent jacket actually repels water or how a non-stick pan keeps your eggs from sticking? It’s often PFASs.
PFASs — poly- and peryfluoroalkyl substances — are a family of more than 4,700 widely-used chemicals found in everything from non-stick cookware to pizza boxes, water-resistant jackets and stain-proof rugs. They were first introduced in the 1950s and widely adopted throughout industry for their ability to repel heat, water, and oil.
Because of their extreme persistence in the environment, Joe Allen, Assistant Professor of Exposure Assessment Science at the Harvard T.H. Chan School of Public Health, dubbed PFASs ‘Forever Chemicals’, a play on their ultra-strong Fluorine-Carbon bond.
PFASs have been linked to immune suppression in children, increased risk of diabetes and obesity, high cholesterol, thyroid disease and low infant birth weight, among other human health risks. Since the early 2000s, major American and international manufacturers have phased out two of the most prevalent PFASs — PFOA (perfluorooctanoic acid) and PFOS (perfluorooctane sulfonic acid) — and replaced them with different members of the PFAS family.
But the replacements are often as toxic as the original.
“It’s like chemical whack-a-mole,” said Elsie Sunderland, the Gordon McKay Professor of Environmental Chemistry at the Harvard John A. Paulson School of Engineering and Applied Sciences, who studies exposure pathways and environmental lifetimes for industrial chemicals and advised Nishizawa’s project. “Once we understand the exposure and health implications of one chemical, a new one pops up.”
From left: Elsie Sunderland, the Gordon McKay Professor of Environmental Chemistry, Nicole Nishizawa, S.B. ‘19, and Andrea K. Tokranov, a graduate student in the Sunderland lab.
While the majority of human exposure to PFAS is thought to come from contaminated food and water, researchers have struggled to quantify how much additional exposure comes directly from manufactured goods and how important this exposure pathway is for populations removed from pollution sources.
“Forever chemicals are clear bad actor chemicals, yet how do we act on that information if we don’t always know which products have them and which ones don’t,” said Allen, who was also involved in Nishizawa’s project. “We need to know where these chemicals are being used and how we are being exposed in our daily lives so that we can make informed decisions – as individual consumers and as an institution.”
Those were the questions that Nishizawa set out to answer.
Nishizawa first got interested in the impact of consumer products on human health her sophomore year when she took ES 161 - Applied Environmental Toxicology with Sunderland. For her final project, Nishizawa and a partner from MIT measured the levels of arsenic in apple juice served in Harvard and MIT dining halls. They found that the levels of arsenic exceeded the regulatory threshold.
“MIT found out about our project and they ended up pulling all of the apple juice from their dining hall services, so that was a really impactful introduction into the field of consumer protection,” said Nishizawa. “It was really inspiring and exciting to know that we were making a difference, even if it was just a small difference.”
The following summer, Nishizawa applied for a grant through the Harvard Office for Sustainability to study PFAS levels in consumer products on campus. Sunderland and her lab had been researching PFAS exposure in drinking water and marine life, but hadn’t explored other types of exposure.
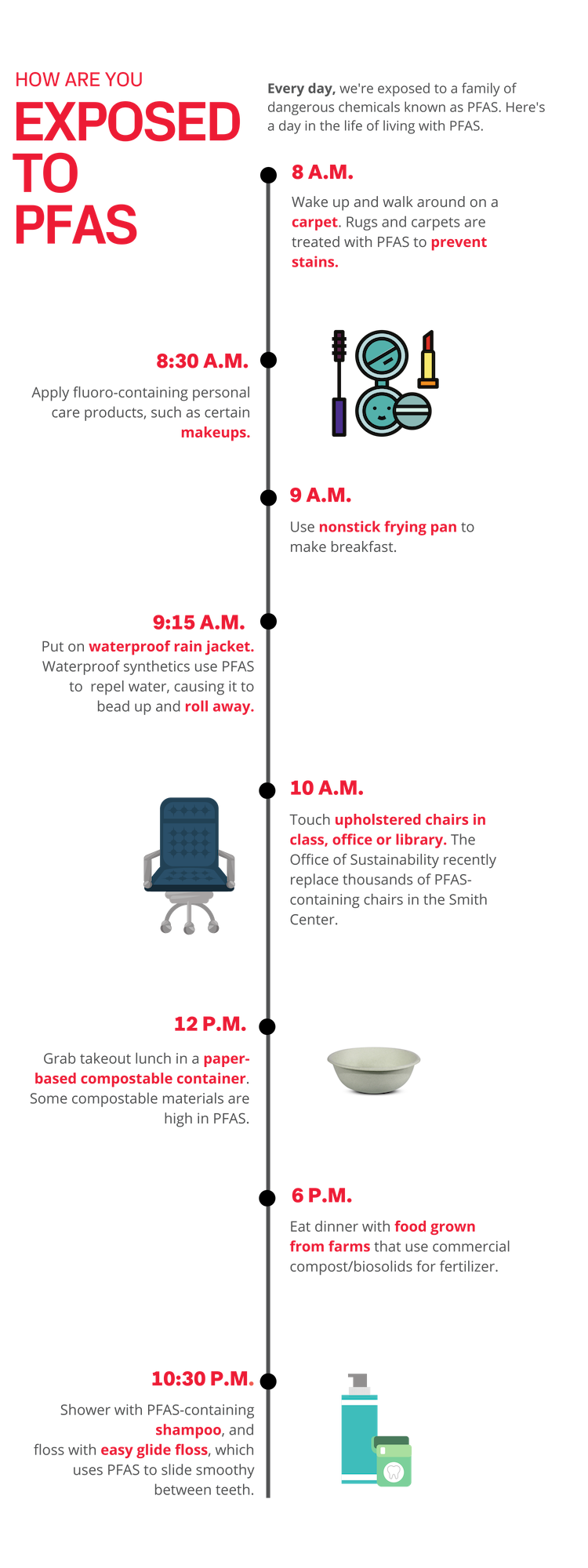
Nishizawa began by collecting the consumer products she personally interacted with on a daily basis: compostable plates and bowls from Harvard dining halls and restaurants, rugs from dorm rooms and offices, shower curtains, a jacket and even an old boot. She collected and tested nearly 100 different samples.
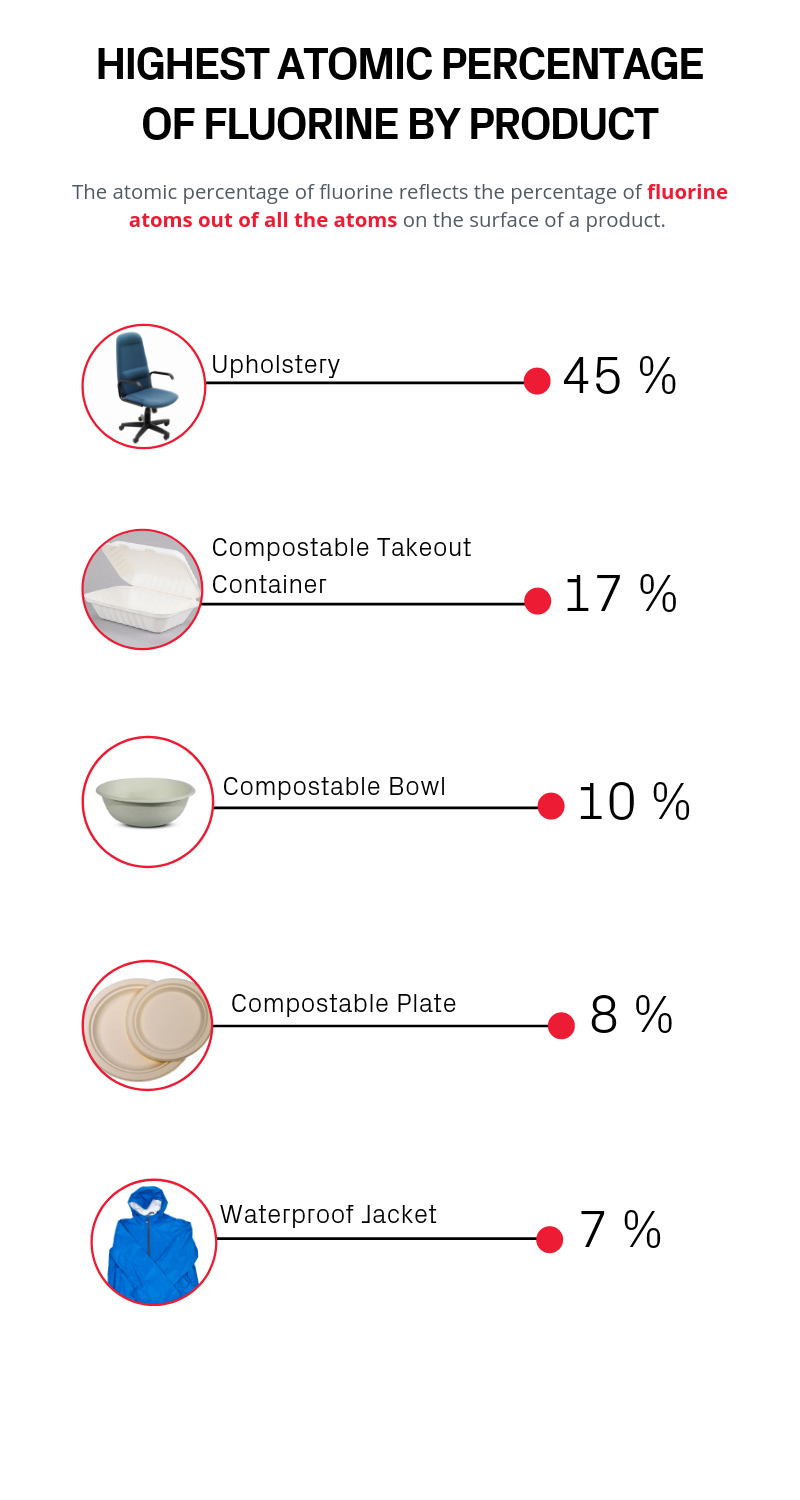
“The first products that we measured were compostable plates and bowls used in the dining halls for Brain Breaks. I used them all the time and they had some of the highest levels of PFAS,” said Nishizawa. “That definitely got me thinking about how often people are exposed to PFAS-laden products, and how I could reduce exposure.”
Paper-based compostable food containers had among the highest PFAS concentrations.
“When I saw the levels of PFAS in some of the compostable containers that we collected, my jaw dropped,” said Andrea K. Tokranov, a graduate student in the Sunderland lab who worked with Nishizawa to test and measure the samples. “You think, this is so environmentally friendly, it's so benign, and then we found huge amounts of PFAS.”
Allen, the founder and director of the Healthy Buildings Program at Harvard, was also surprised by the results -- especially since a rug cutting from his own office was among the samples with the highest levels of PFAS.
“There is nothing like seeing an actual test result to take the invisible and make it visible,” said Allen. “It makes you look at the world around you a bit differently.”
During the project, Tokranov and Nishizawa developed a new method to measure PFAS levels in consumer products that is easier and more widely available than previous methods.
“Because there are thousands of different PFAS structures, being able to quantify all of them is nearly impossible,” said Tokranov. “Traditional methods to measure PFAS only capture a small fraction of the fluorinated substances present in consumer products.”
So, Carlo Alberto Amadei, another SEAS graduate student working with Tokranov and Sunderland, suggested turning to a common piece of lab equipment — the X-ray photoelectron spectroscopy machine (XPS). The XPS is really good at measuring surfaces, which is the most likely point of exposure to PFAS in commercial goods. By using the XPS to etch the material at different surface depths, the researchers were able to build a holistic profile of PFAS levels in the material.
The research was published in Environmental Science and Technology Letters.
“XPS has been around for a long time and has been used to measure PFASs before, but no one ever used it to measure PFASs in consumer products,” Tokranov said. “Now that we know we can use the XPS to accurately measure PFAS levels, the next question is what can it tell us about human exposure.”
Nishizawa and Tokranov’s results shed light on the avenues of exposure right here on campus.
“This research is a perfect example of how Living Lab research done at Harvard helps inform the decisions that are being made here on campus,” said Allen.
In fact, Nishizawa and Tokranov’s results are helping Harvard make better purchasing decisions, said Heather Henriksen, the Managing Director of the Office of Sustainability.
“This is the power of science,” said Henriksen. “You publish peer-reviewed science that enables big owners and buyers, like Harvard, to immediately shift their purchasing power. When you have the science, you can make clear market signals to producers, who want to give the customer what they want.”
Henriksen is leading the Healthier Building Materials Academy at Harvard, which is working to bring the University’s project management and purchasing community together with vendors to reduce the use of certain chemicals, including PFAS, in the University’s capital projects.
Nishizawa’s research has highlighted several high-impact areas for the Healthier Building Materials Academy.
“Having the Office of Sustainability use their resources to leverage change from our data is really exciting,” said Nishizawa.
“The greatest thing that we produce as a university is our science and our students. If we can train our students to use their research to make a real change in the world, that is our greatest accomplishment,” said Henriksen.
For her senior thesis, Nishizawa extended her research into PFAS exposure to include how toddlers are exposed to the chemicals from carpets in daycare centers. Toddlers are particularly vulnerable to PFOS and PFOA exposure because they are in such close contact with carpets and often ingest dust containing PFAS. Nishizawa found that when exposed to high dust concentrations, toddlers at daycare centers may be at risk of exceeding EPA Minimum Risk Levels for PFAS. However, by reducing PFAS- containing products, decreasing dust levels in indoor spaces, and spending less time inside, toddlers can have reduced PFAS exposure via dust ingestion.
Topics: Environment
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu