News
Contact:
Steve Bradt
617-496-8070
CAMBRIDGE, Mass. - Dec. 17, 2009 - Scientists at Harvard University have used light and genetic trickery to trace out neurons' ability to excite or inhibit one another, literally shedding new light on the question of how neurons interact with one another in live animals.
The work is described in the current issue of the journal Nature Methods. It builds upon scientists' understanding of the neural circuitry of the nematode worm Caenorhabditis elegans, frequently used as a model in biological research. While the detailed physical structure of C. elegans' scant 302 neurons is well documented, the new research helps measure how neurons in this organism affect each others' activity, and could ultimately help researchers map out in detail how neural impulses flow throughout the organism.
"This approach gives us a powerful new tool for analyzing small neural circuits, and directly measuring how neurons talk to each other," says Sharad Ramanathan, an assistant professor of molecular and cellular biology and of applied physics at Harvard. "While we've only mapped out the interplay of four neurons, it's the first time scientists have determined the ability of multiple neurons in a circuit to excite or inhibit their neighbors."
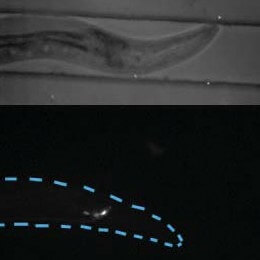
Zengcai Guo and Ramanathan combined genetically encoded calcium sensors and light-activated ion channels with optics. The scientists used a mirror array to excite individual neurons -- each just two to three millionths of a meter wide -- while simultaneously measuring calcium activity in multiple other neurons. This calcium activity serves to indicate whether these other neurons were activated or inhibited by the neuron that was primed with a burst of light.
"Using this technique, for the first time, we could excite both a sensory neuron and an interneuron and monitor how activity propagates," says Guo, a research assistant in Harvard's Center for Systems Biology, Department of Molecular Biology, and School of Engineering and Applied Sciences. "We expect that our technique can eventually be used more broadly to measure how activity propagates through neural circuits."
Manipulating neurons with light, Guo and Ramanathan were able to evoke an avoidance response -- causing the worm to back away from light -- that is normally prompted only when the organism is touched.
With a compact nervous system consisting of only 302 neurons linked by some 7,000 synapses, the nematode C. elegans is an ideal system for studying the interplay between neural circuits and behavior. While the physical connectivity of the neurons in this nematode is well known, scientists know very little about which of these connections are excitatory and which are inhibitory.
Because of the small sizes of the neurons and a tough cuticle surrounding the worm, electrophysiological recordings can be made from only one neuron at a time, precluding the possibility of any circuit-level analysis of neural activity. By establishing this first fully genetically encoded light-based electrophysiology, the authors have developed a way to overcome this limitation.
Guo and Ramanathan's Nature Methods paper was co-authored by Anne C. Hart of Massachusetts General Hospital and Harvard Medical School. Their work was funded by the National Institute of General Medical Sciences.
###
A lite-1 worm expressing ChR2 in ASH rapidly initiated reversals upon blue light illumination. The movie begins with illumination with low intensity diffuse white light. The duration of blue light illumination is indicated by 'blue light on'. We note that the worm rapidly reversed its direction of movement in response to blue light illumination and continued to move forward, in a different direction after an  turn. The light intensity used was about 1 mW mm-2. The light response of the same transgene in wild type genetic background is similar.
turn. The light intensity used was about 1 mW mm-2. The light response of the same transgene in wild type genetic background is similar.
Specific stimulation of ASH activates the command interneurons AVA and AVD in a lite-1 worm. The movie shows the same individual as shown in Figure 5a. The ASHR neuron was stimulated using blue light for about 10 seconds and the responses in ASHR, AVAR, AVDR, and ASIR were monitored using low intensity 488nm laser. The fluorescence intensity in ASHR increased upon blue light stimulation. The fluorescence intensity in ASIR changed little, indicating that the stimulation of ASHR was specific. The fluorescence intensity in AVAR and AVDR increased following that of ASHR, indicating that AVAR and AVDR were activated due to ASHR activation. The jump of fluorescence intensity in ASHR when the stimulation light was turned on is explained in Fig. 4b and Supplementary Fig. 18. The movie was prepared in ImageJ for microscopy (Version 1.41a). Specifically, the 16 bit raw images (in TIFF format) were first changed into 8-bit format. Images are color coded (using 'Green Hot' option) with green indicating low fluorescence and yellow-white high fluorescence. The stimulation duration is indicated using 'Stimulation on'.
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.