News
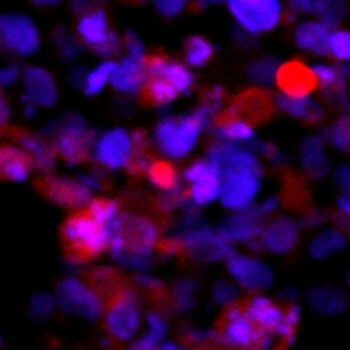
Wyss Institute scientists have designed a model for assessing how extracellular matrix stiffness affects cancer treatment. In this image, human leukemia cells are encapsulated in the team's novel 3D hydrogel that mimics natural tissues. (Credit: Wyss Institute at Harvard University.)
(BOSTON) - Chemotherapy is often used to combat malignant tumors, but rarely completely cures patients due to cancer cells’ resistance to drugs. It has been thought that the environment in which particular cancer cells live could impact their response to specific drugs, but until now, it’s been difficult to analyze exactly how mechanics—specifically, stiffness of the extracellular material that surrounds cells and structures tissues—alter a drug’s efficacy.
Harvard scientists have now found a way to analyze how the stiffness of this “extracellular matrix” affects chemotherapy treatment. David Mooney, the Robert P. Pinkas Family Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), and Core Faculty member at the Wyss Institute for Biologically Inspired Engineering, is the senior author on the paper that details the novel method, published today in the Proceedings of the National Academy of Sciences report.
Working with Jae-Won Shin, Mooney has unveiled a new drug screening assay using alginate hydrogels — biocompatible materials made from a polysaccharide found in brown seaweed — which can be tuned to recapitulate mechanical stiffness and other physical properties that are characteristic within tumor and normal tissue.
“To have success with chemotherapy and other drug therapies, we will likely need to screen their effectiveness against cells living in various environments, and not just assume that cells will always respond to a drug the same way that they would in conventional cell culture,” said Mooney.
Mooney’s team has previously analyzed and confirmed the importance of mechanical cues by developing tunable alginate hydrogels for applications such as tissue regeneration, stem cell differentiation, stimulation of blood vessel formation, and bone and cartilage repair.
Now, Mooney and Shin have designed a 3D microenvironment with tunable matrix stiffness, inside of which cancer cells can be bombarded with drugs and their resistance (or destruction) observed. By varying matrix stiffness, they demonstrated that mechanical cues control how cancer cells respond to chemotherapy drugs. For many drugs, the softer the matrix, the more that cancer cells resisted treatment.
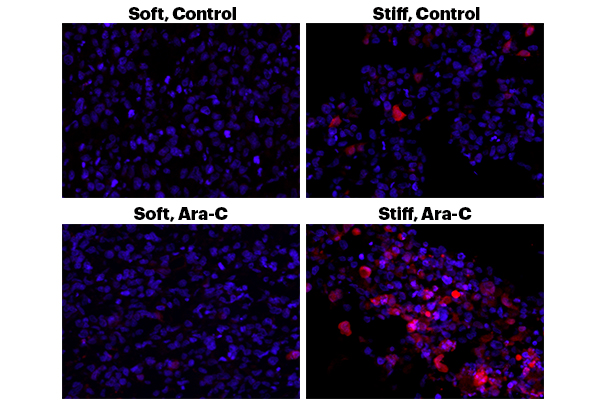
Wyss Institute scientists have designed a model for assessing how extracellular matrix stiffness affects cancer treatment. In these images, human leukemia cells are encapsulated in the team's novel 3D hydrogel that mimics natural tissues. Above, the appearance of red or pink color indicates leukemia cell death, showing that a stiffer microenvironment results in more cancer cell death. Below, the effects become much more exaggerated when the models are inundated with the chemotherapy drug known as 'Ara-C'. Leukemia cells in a stiffer matrix are killed at a much higher rate than in the softer matrix, where the lack of red color indicates resistance to chemotherapy. (Credit: Wyss Institute at Harvard University.)
Using this approach, a wide array of different cellular environments mimicking various tissues in the body could be used to more realistically screen cancer cells for their responses to potential drugs, allowing the right drug regimen to be selected for treating a specific patient’s type of cancer and its location.
Mooney and Shin implanted the same hydrogels in mice to investigate in vivo if matrix softening would accelerate cancer growth and increase its resistance to chemotherapy – and indeed, it did. In the future, 3D hydrogels could better identify promising drugs early in the discovery process and, much farther down the pipeline, potentially be used to design personalized cancer drug therapy to overcome resistance.
“We envision that our 3D hydrogels could bridge the gap between in vitro drug screening and in vivo pre-clinical studies,” said Shin, who was formerly a Wyss Institute Postdoctoral Fellow and is currently Assistant Professor of Pharmacology and Bioengineering at University of Illinois at Chicago.
Although Mooney and Shin have initially zeroed in on using the method to improve cancer treatment, it could be used more broadly to screen drugs for their efficacy in treating a number of diseases throughout the body.
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu