News
Professor Howard Stone explained the science involved in the formation of bubbles during the annual SEAS Holiday Lecture on Dec. 18. (Photo by Eliza Grinnell/SEAS Communications.)
Drop a paperclip into a glass of water and it will quickly sink to the bottom. But gently set that same paperclip onto the water’s surface and it will float.
Why does the paper clip behave differently? The contrasting results are caused by surface tension, explained Professor Howard Stone during the 15th annual Holiday Lecture at the John A. Paulson School of Engineering and Applied Sciences on Dec. 18.
Surface tension occurs at the interfaces of water and air because water molecules have greater attraction to each other than air molecules, causing an inward force to be exerted across the surface of the liquid, which behaves like it is covered with a thin membrane.
The paperclip experiment was one of several interactive demonstrations utilized by Stone, a former member of the Harvard faculty and now Dixon Professor in Mechanical and Aerospace Engineering at Princeton University, to showcase the power of science to hundreds of local children and their parents.
Stone, along with research assistant Daniel Rosenberg, blew bubbles, popped a water balloon, compared the sizes of water and ethanol droplets, floated a piece of string across the surface of water, and showed slow-motion videos of water striders in action – all to explain the effects of surface tension.

Stone (left) and Rosenberg pop a water balloon to show how surface tension and gravity impact the shape of the water as it falls. (Photo by Eliza Grinnell/SEAS Communications.)
That surface tension is disrupted, causing the paper clips in Stone’s experiment to drop to the bottom of the water tank, if just a few drops of soap are added to the water.
The soap film that forms on the surface of water is another example of how forces create unique properties at interfaces, Stone explained. Soap molecules are composed of hydrophilic and hydrophobic components; the hydrophobic components push their way through water molecules to escape, forming a film with two rows of soap molecules separated by a thin layer of fluid.
“We think about the soap film as a tug of war,” he said. “The entire film is under pressure and pulling on itself.”
Kids in the audience were called upon to illustrate this chemical process. At the front of the lecture hall, children wearing t-shirts labeled either hydrophobic or hydrophilic, playing the parts of soap molecules, lined up on either side of children wearing blue shirts, who signified water molecules. Grasping hands, the human soap film snaked across the front of the lecture hall, eliciting cheers from the parents in their seats.
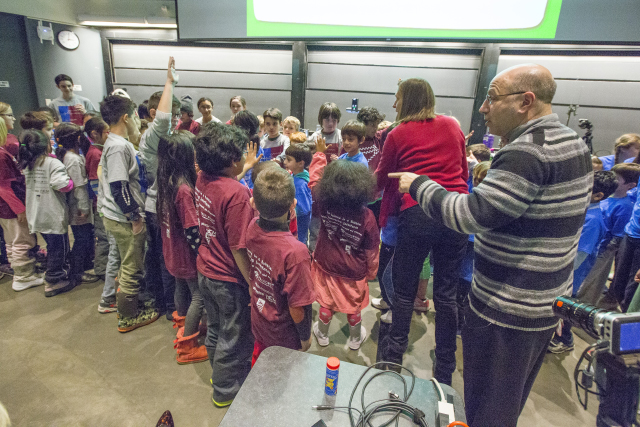
Stone directs the children as they act out the formation of a soap film. (Photo by Eliza Grinnell/SEAS Communications.)
Being able to act out a chemical reaction was the most fun part of the lecture for 9-year-old Rachel Liu. She was especially interested to learn about the hydrophilic and hydrophobic components of soap molecules.
“Science tells us a lot about the world around us,” she said.
After hearing Stone’s presentation, 8-year-old Walter Hopwood said he will no longer take soap bubbles for granted. He was impressed by how Stone and Rosenberg used “cool experiments” to explain each process.
Giving children a basic understanding of scientific principles, and encouraging them to stay curious, is the goal of the lectures, Stone said. He drew inspiration from the book “Soap Bubbles,” published in 1890 and still in print today. It is based on a collection of public lectures on the properties of soap films that British physicist Charles Vernon Boys presented to families in London during the late 1800s.
“I hope these kids realize that there are always more things to learn,” Stone said. “Keep asking questions, thinking deeply, and making your own observations. There are so many fascinating things in the world.”

Nine-year-old Grace Liu watches intently as Stone plays a video of a water strider moving across the water's surface. (Photo by Eliza Grinnell/SEAS Communications.)
The Holiday Lecture, jointly presented by SEAS and the Princeton University School of Engineering and Applied Sciences, was sponsored by the Materials Research Science and Engineering Center (MRSEC) at Harvard, the Princeton Center for Complex Materials, the Princeton University MRSEC, and the Princeton Institute for the Science and Technology of Materials.
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Press Contact
Adam Zewe | 617-496-5878 | azewe@seas.harvard.edu
