News
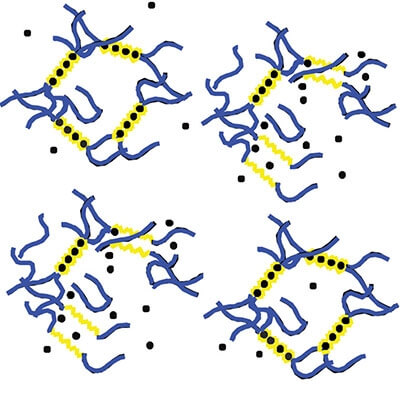
Harvard researchers have shown how ultrasound can temporarily disrupt an alginate hydrogel, which is held together by calcium ions (black dots) that are bound to acid chains (yellow). The gel self-heals when the ultrasound stops. (Image credit: Wyss Institute and Harvard SEAS.)
Cambridge, Mass. – June 23, 2014 – Current drug delivery systems used to administer chemotherapy to cancer patients typically release a constant dose of the drug over time—but a new study challenges this “slow and steady” approach and offers a novel way to locally deliver the drugs “on demand,” as reported in the Proceedings of the National Academy of Sciences (PNAS).
Led by David J. Mooney, the Robert P. Pinkas Family Professor of Bioengineering at the Harvard School of Engineering and Applied Sciences (SEAS) and a Core Faculty member at Harvard’s Wyss Institute for Biologically Inspired Engineering, the research team loaded a biocompatible hydrogel with a chemotherapy drug and used ultrasound to trigger the gel to release the drug. Like many other injectable gels that have been used for drug delivery for decades, this one gradually releases a low level of the drug by diffusion over time. To temporarily increase doses of drug, scientists had previously applied ultrasound, but that approach was a one-shot deal as the ultrasound was used to destroy those gels.
This gel was different.
The team used ultrasound to temporarily disrupt the gel such that it released short, high-dose bursts of the drug—akin to opening up a floodgate. But when they stopped the ultrasound, the hydrogels self-healed. By closing back up, the gels were ready to go for the next “on demand” drug burst, providing an innovative way to administer drugs with a far greater level of control than was possible before.
That’s not all. The team also demonstrated—in lab cultures and in mice with breast cancer tumors—that the pulsed, ultrasound-triggered hydrogel approach to drug delivery was more effective at stopping the growth of tumor cells than traditional, sustained-release drug therapy.
“Our approach counters the current paradigm of sustained drug release and offers a double whammy,” said Mooney. “We have shown that we can use the hydrogels repeatedly and turn the drug pulses on and off at will, and that the drug bursts in concert with the baseline low-level drug delivery seem to be particularly effective in killing cancer cells.”
The advance holds promising implications for improved cancer treatment and other therapies requiring drugs to be delivered at the right place and the right time—from post-surgery pain medications to protein-based drugs that require daily injections. It requires an initial injection of the hydrogel, but the approach could be a much less traumatic, minimally invasive and more effective method of drug delivery overall, Mooney said.
“We want to give clinicians the ability to deliver drugs as locally as possible, combined with the flexibility to temporally control the dose,” said co-lead author Nathanial Huebsch, Ph.D. ’10, who was a Harvard SEAS graduate student in the Harvard-MIT Division of Health Sciences and Technology at the time of the research and is now a postdoctoral fellow at the Gladstone Institute of Cardiovascular Disease in San Francisco. For example, many cancer patients require a regular dose of pain killers, but unpredictable pain attacks require them to take much larger doses over a short time.
Key to the team’s success in designing a hydrogel that self-heals is choosing the right kind of hydrogel with the right kind of drug—and applying the right intensity of ultrasound.
“We were able to trigger our system with a level of ultrasound that was much lower than high-intensity focused ultrasound that is used clinically to heat and destroy tumors,” said co-lead author Cathal Kearney, who was a postdoctoral fellow at SEAS at the time of the study. He is now a senior research fellow at the Royal College of Surgeons in Ireland. “The careful selection of materials and properties make it a reversible process,” Kearney said.
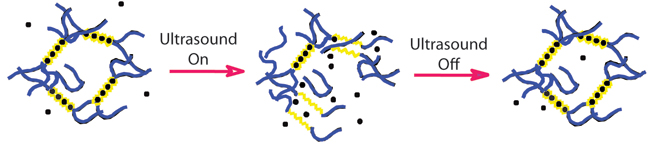
This graphic shows how ultrasound can temporarily disrupt an alginate hydrogel, which is held together by calcium ions (black dots) that are bound to acid chains (yellow). The gel self-heals when the ultrasound stops. (Image credit: Wyss Institute and Harvard SEAS.)
The team carried out the majority of their work for this study with a gel made of alginate, a natural algae-derived polysaccharide that is held together with calcium ions. In a series of laboratory tests they found that with the right level of ultrasound, the bonds break up and enable the gel to release its drug cargo—but as long as the gel is in the presence of more calcium, the bonds reform and the gels self-heal.
Once the team knew the gel would self-heal, they tested out a drug they suspected it would hold well—in this case a chemotherapy drug called mitoxantrone, which is often used to treat breast cancer. Sure enough, the ultrasound triggered the gel to release the blue-colored drug, as indicated by the newly blue color of the surrounding medium. Just a single ultrasound dose was effective, and the gel reformed after it was disrupted, making multiple cycles possible.
Next, they tested the treatment on mice that had human breast cancer tumors implanted in their bodies. They injected the drug-laden gel close to the tumors, and over the course of six months the mice that received a low-level sustained release of the drug with a daily concentrated pulse of ultrasound (just 2.5 minutes) fared significantly better than mice treated the same but without ultrasound. In contrast to the other groups, the tumors in the ultrasound-treated mice did not grow substantially and the mice survived for an additional 80 days to boot.
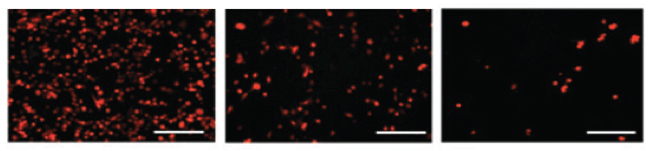
Fluorescence micrographs show red-colored breast cancer cells that were exposed to three different treatments. The use of ultrasound to trigger periodic bursts of higher drug doses (right) was most effective in killing the cancer cells. (Image credit: Wyss Institute and Harvard SEAS.)
“These results demonstrate how applying novel engineering approaches and programmable nanomaterials can create entirely new solutions to critical medical problems,” said Don Ingber, founding director of the Wyss Institute who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children's Hospital, and Professor of Bioengineering at Harvard SEAS. “Dave’s work shows that these new responsive hydrogels that remodel reversibly when exposed to ultrasound energy at the nanoscale not only provide a new way to administer drugs on demand; they also produce better responses to therapy even in a disease as difficult to treat as cancer.”
This advance to use simple ultrasound pulses and readily available hydrogels in a new way comes on the heels of Mooney’s work using low-power lasers to trigger stem cells to regenerate the material that makes up teeth. The team also demonstrated that the gel can release other kinds of cargo as well, including proteins, which lays the groundwork for potentially using these hydrogels for tissue regeneration, and condensed plasmid DNA, suggesting their potential use in gene therapy.
The researchers plan to explore these other potential applications, as well as the possibility of unleashing two different drugs independently from the same hydrogel, said Mooney.
This work was funded by the Materials Research Science and Engineering Center at Harvard University, a California Institute of Medicine Fellowship, and the National Institute of Dental and Craniofacial Research, which is one of the National Institutes of Health (NIH).
Topics: Materials, Bioengineering
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Press Contact
Caroline Perry