News
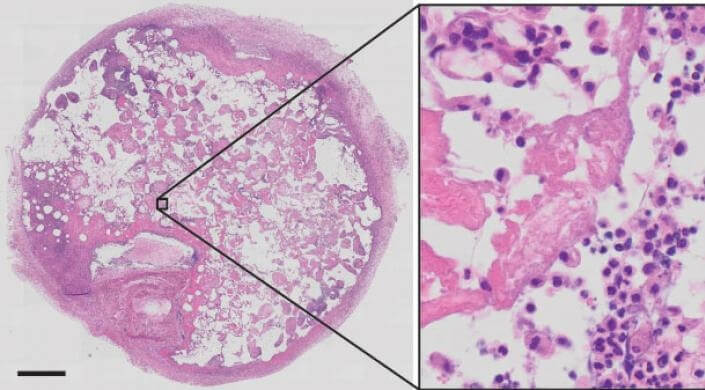
This is a histological section of a retrieved beta cell scaffold stained for cellular structures 14 days after implantation. In the magnified area on the right, the nuclei of trapped autoimmune T cells are showing up as dark dots. (Image courtesy of Harvard University)
Early in embryological development, the immune system learns not to attack the body’s own cells. In many autoimmune diseases, including type 1 diabetes, multiple sclerosis, and rheumatoid arthritis, this self-tolerance is ineffective or reversed partly because of rogue T cells. However, it can be difficult to access and study these disease-initiating T cells because only about 1 in 100,000 circulate in the blood.
Researchers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) and the Wyss Institute for Biologically Inspired Engineering report that by mimicking the insulin-producing environment of the pancreas that is destroyed in type 1 diabetes, they can recruit, isolate and characterize diabetes-causing immune cells in a mouse model of the disease. The team also includes researchers from the Joslin Diabetes Center at Harvard Medical School.
Their biomaterials approach, published in the journal Diabetes, could be the basis for similar studies in human patients and also could be used to investigate the causes of other autoimmune diseases.
The little understood class of autoimmune T cells that are at the root of type 1 diabetes are mostly found in inaccessible pancreas tissue where they wreak havoc on small islets of insulin-producing beta cells.
“We simulated these inflamed islets of pancreatic beta cells with a biomaterial scaffold right under the skin of mice,” said Omar Ali, former staff scientist in the Advanced Technology Team at the Wyss Institute and co-author of the study. “By presenting beta cell antigens, we hoped to use the scaffold as an autoimmune T cell trap, recruiting and capturing the T cells so we could harvest them in vivo.”
By presenting beta cell antigens, we hoped to use the scaffold as an autoimmune T cell trap, recruiting and capturing the T cells so we could harvest them in vivo.
Ali led the study with Thomas Serwold, an Investigator at the Joslin Diabetes Center’s Section on Immunobiology.
To generate the pancreas-mimicking biomaterial, the researchers infused biological components from a beta cell-derived cell line into a macroporous, biodegradeable scaffold. When implanted under the skin of a diabetic mouse, the trap recruits autoimmune T cells by inducing them to move through its pores into its interior where they bind self-antigens that are part of the molecular repertoire of the beta cells and expand their numbers. After retrieval of the scaffold, the autoimmune T cells can be easily isolated and further investigated.
“Our study provides proof-of-principle that the trapped autoimmune T cells share genetic and functional features with the autoimmune T cells residing in the inflamed pancreas, and that they indeed can promote diabetes when transferred to a mouse that hadn’t developed the disease yet,” adds Ali.
The researchers believe that by developing analogous injectable scaffold systems for humans that can be easily harvested through needle biopsies, they will be able to analyze what makes diabetes-causing autoimmune T cells genetically and functionally different from normal T cells.
“Our work’s underlying concept could also give us a handle on autoimmune T cell populations in other diseases and could be used as a read-out for the efficacy of drugs that may actually raise immune tolerance in these disorders,” said co-author David Mooney, the Robert P. Pinkas Family Professor of Bioengineering at SEAS and Wyss Institute Core Faculty member.
The idea for autoimmune T cell-trapping biomaterials was inspired by a ‘cancer vaccine’ that was previously engineered by Mooney’s team at SEAS and the Wyss Institute, which is designed to attract and re-educate immune cells in tumor patients to direct their destructive abilities against tumor tissue.
Cutting-edge science delivered direct to your inbox.
Join the Harvard SEAS mailing list.
Press Contact
Leah Burrows | 617-496-1351 | lburrows@seas.harvard.edu